Cationic polymerization refers to the general term for polymerization reactions initiated by cations, which have the advantages of fast curing speed, high efficiency, and low energy consumption, and are a green and environmentally friendly curing method that is widely used in coatings, inks, adhesives, electronic packaging, and other fields.
Cycloaliphatic epoxy resins are very easy to undergo cationic polymerization due to their special chemical structure, compared with ordinary bisphenol A epoxy. The reaction mechanism is as follows:
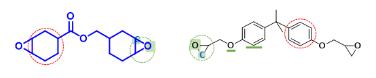
The reaction process of cationic epoxy polymerization is mainly divided into three stages: chain initiation, chain growth, and chain termination. Here's an example of the mechanism of cationic polymerization of cycloaliphatic resin using diphenyliodonium salt as an initiator:
The initiator decomposes under irradiation or heating conditions to produce cations, free radicals, and free radical-cation pairs. Among them, cations and free radical-cation pairs react with monomers or solvents in the reaction mixture to generate proton acids. The proton acid initiates cationic ring-opening polymerization of epoxide monomers. The specific process is shown in the following figure:
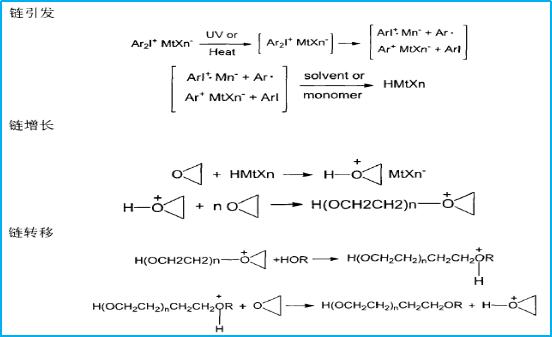
Cationic polymerization can be initiated by either light or heat. Except for the different initiation conditions, the differences in initiator selection and reaction mechanism between these two initiation methods are basically the same. Here, we discuss the thermal initiation of cationic polymerization. Thermal initiation of cationic polymerization is not limited by the light source, avoiding constraints in terms of sample size, thickness, shape, and is widely used in adhesives, electronic packaging, and other fields.
The thermal initiation system of cationic polymerization mainly consists of monomers, initiators, and initiation conditions (heat sources). In this system, cycloaliphatic epoxy resins can mainly be selected as monomers. Additionally, the type, amount, and change of initiators and initiation conditions will affect the polymerization rate. Generally, the addition amount of the initiator is 0.1-2 wt%, and within this range, as the addition amount of the initiator increases, the reaction rate increases. However, if the amount of the initiator is too high, not only will there be no significant improvement in the reaction rate, but it will also affect the performance of the cured product, such as yellowing, deformation, and brittleness. Increasing the reaction temperature will speed up the curing rate, and the longer the heating time, the more complete the curing.
This issue conducts correlation performance evaluation experiments on Jiangsu Tetra's typical representative product TTA21 and thermal-activated cationic initiator, and compares it with the anhydride curing system and bisphenol A epoxy system. The anhydride chosen is Methyl Hexahydrophthalic Anhydride (MHHPA), and the bisphenol A epoxy chosen is the EP128 resin.
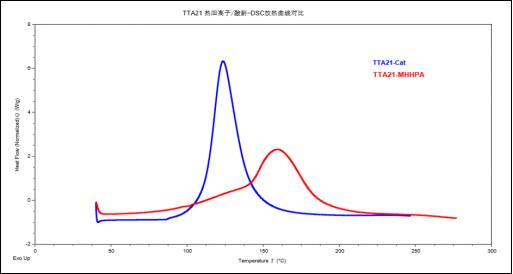
Figure 1. Comparison of DSC heat release curves of TTA21 thermal cationic/anhydride system
From Figure 1, we can see that the reaction heat of TTA21 thermal cationic curing is concentrated, and the onset temperature and peak temperature of heat release are both low, indicating that TTA21 thermal cationic curing has higher reaction activity than anhydride curing, and can complete the curing reaction at lower temperature and in a shorter time.
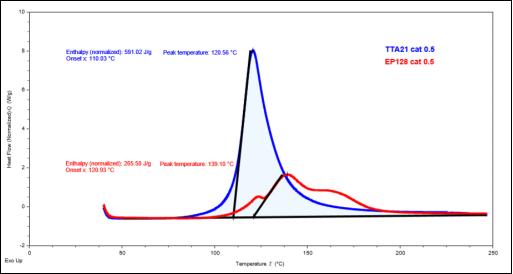
Figure 2. Comparison of DSC heat release curves of TTA21 /EP128 thermal cationic curing
From Figure 2, it can be seen that TTA21 is much higher than EP128 in terms of thermal cationic reaction activity, indicating that cycloaliphatic epoxy is more suitable for thermal cationic curing, while the thermal cationic reaction activity of bisphenol A epoxy is low, and due to its high viscosity, it is not suitable for thermal cationic curing in actual production applications.
The following Table 1 shows the basic performance data of TTA21 and EP128 in thermal cationic curing. This demonstrates that TTA21 is suitable for thermal cationic curing while EP128 is not suitable for thermal cationic curing.
Table 1. Basic performance data comparison of TTA21 and EP128 in thermal cationic curing
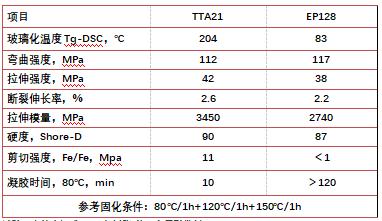
The basic composition of the test formula is: epoxy resin/thermal cationic initiator = 100/0.5
In summary, cycloaliphatic epoxy resin TTA21(3 4 epoxycyclohexylmethyl 3 4 epoxycyclohexanecarboxylate Cas 2386-87-0) is suitable for thermal cationic curing due to its special chemical structure, high reaction activity, fast curing speed, high adhesive strength, and excellent curing performance.